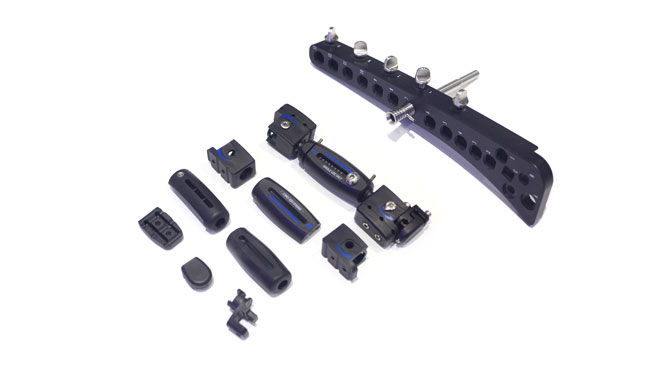
PEEK surgical head frame and retractor system is generally larger overall structure, if the use of metal will be particularly bulky, the doctor's surgical operation is very inconvenient, the use of PEEK material can reduce the weight of 80%, greatly reducing the weight of the instrument, greatly improving the doctor's surgical lightness and flexibility.
PEEK(poly ether ether ketone) is confirmed biocompatibility material three decades ago.By the late 1990s, medical grade PEEK polymer had emerged as the leading high-performance thermoplastic candidate for replacing metal implant components, especially in orthopedics and trauma devices.Unfilled PEEK biomaterials can exhibit an elastic modulus ranging between 3 and 4 GPa, the modulus can be tailored to closely match cortical bone (18 GPa) or titanium alloy (110 GPa) by preparing carbon fiber-reinforced (CFR) composites with varying fiber length and orientation.Medical PEEK is increasingly used as biomaterials for the medical products.
Plus, for medical device parts,PEEK provides a great benefit: It saves weight,allows more design freedom and a greater functional integration, it also scores with X-ray transparency and elasticity which corresponds approximately to bone.
PEEK(Poly ether ether ketone) is the most well known and important member of the PAEK(Poly aryl ether ketone) group. This high-performance polymer material combines high-termperature resistance,good wear, and excellent mechanical properties. For instance, the glass transition temperature of PEEK is around 143℃ (289℉) ,and melts around 343℃ (662℉). The carbon fiber filled PEEK or glass fiber PEEK material have a useful operating temperature of up to 250℃ (482℉).
PEEK material is the best comprehensive performance of special engineering plastics. PEEK shows excellent overall performance due to its large amount of benezene ring structure in its molecule. At present, medical grade PEEK material is widely used in high-end medical device.
PEEK and PPSU is used in many different areas of the medical industry, from implantable devices that have to be both strong and biocompatible to reusable medical instruments that have to be sterilized repeatedly using harsh processes such as autoclaving. PEEK and PPSU is an good choice for medical applications for numerous reasons, including its excellent fatigue/wear properties, high strength-to-weight ratio, and biocompatibility.

Polyether Ether-Ketone (PEEK) is an engineering grade thermoplastic polymer with characteristics that are uniquely suited to many medical tubing applications. PEEK is a high purity, semi-crystalline, organic polymer with excellent resistance to thermal degradation at high temperatures. PEEK tubing exhibits high column strength, as well as high tensile strength and high flexural modulus, making it perfect for catheters and other tubing applications requiring good torque response and pushability.
Extruded PEEK tubing is used in medical applications where exceptionally high rigidity is needed. Due to PEEK’s excellent biocompatibility, it is a suitable choice for long-term implants. With a modulus similar to bone, PEEK is commonly used in orthopedic implant applications. PEEK tubing can also be found in medical applications requiring high vacuum or pressure holding capability. The material can serve as an effective alternative to steel, aluminum, glass, and other polymers, including use as a composite structure in reinforced catheter shafts.

30% Carbon Filled PEEK has been reinforced with carbon fibers that improve PEEK’s compressive strength and stiffness, while also reducing its expansion rate. This grade of PEEK offers the best wear resistance and load bearing capabilities, while also offering 3.5 times higher thermal conductivity than Unfilled PEEK. This improves bearing life and capability. 30% Carbon Filled PEEK ( PEEK5600CF30) is available in sheet and rod in a black color. PEEK5600CF30 is also can be made the injection Trauma PEEK Targeting Device.
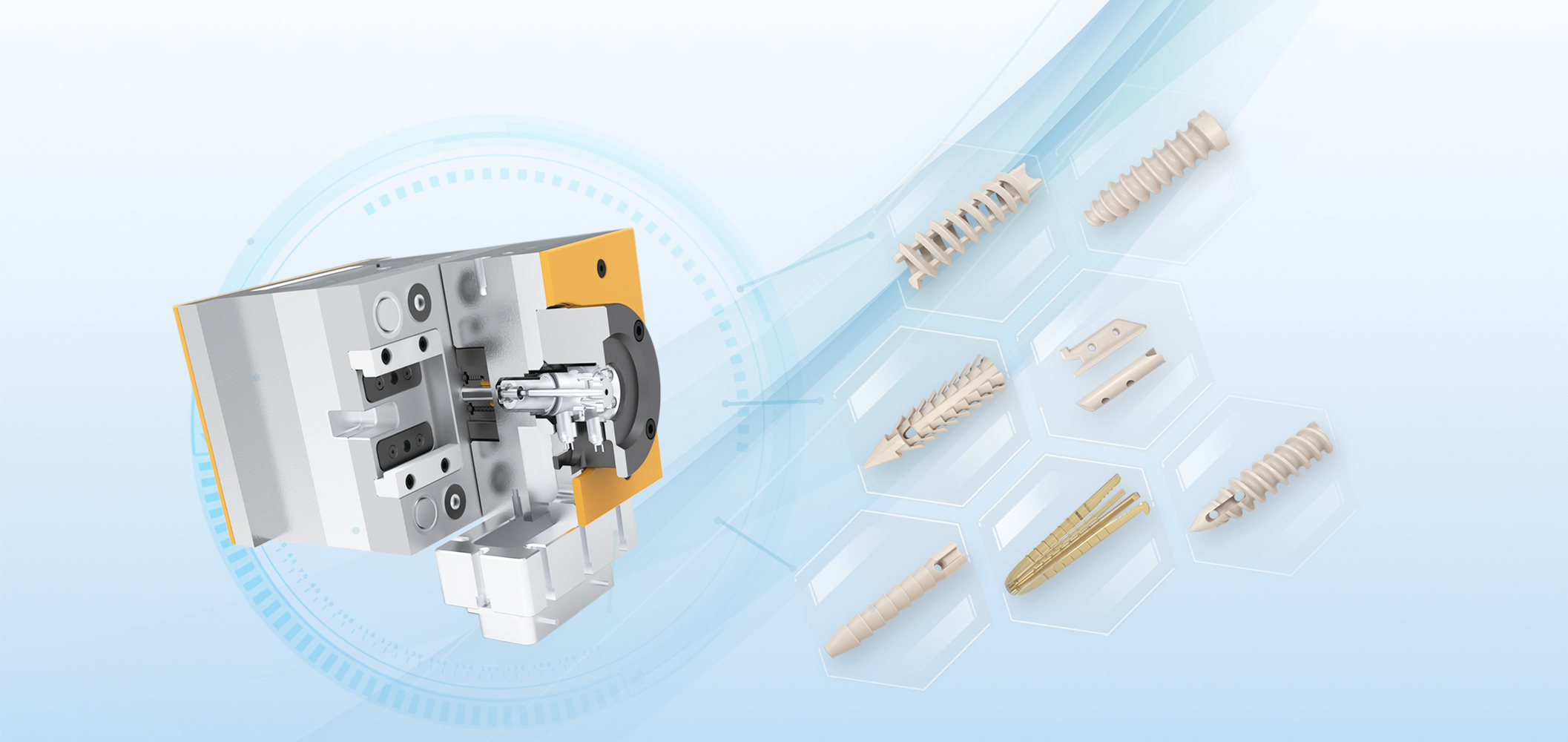
As the fifth category of orthopedics in China, sports medicine has been widely used in orthopedics with its two core characteristics of minimally invasive surgery and reduction of soft tissue damage.The market share of the global orthopaedic industry is as high as over 1%, with a rapid growth rate of 30% every year, which is the fastest growing segment of the orthopaedic industry in the future.At present, more than 90% of the market share of sports medicine is occupied by foreign medical giants. At present, medical transport in China is still in the early stage of development.However, with the development of domestic orthopedic industry, the replacement of import is an inevitable trend.In the next 3-5 years, domestic sports medicine will focus on the development of registration, involving mold opening and injection molding foundry, and the demand for implant-grade particles will also increase rapidly. There are huge demands and opportunities.
JUNDE Medical is always at the forefront of the market. Since the middle of 2020 year, JUNDE PEEK has started to work with dozens of trauma device manufactures to develop the implantable intramedullary nail. The implantable PEEK material is provided by the medical partner, and JUNDE company provide the OEM service.

AKSOPEEK is the registrated brand for implantable PEEK.The authority license is under the process and it will be gained in the end of 2022 as plan. AKSO is the representative of the goddess of health in ancient Greek mythology. AKSO is the medical grade long-termed implantable PEEK by JUNDE medical.Four kinds of implantable PEEK will be available,
AKSOPEEK Natural、AKSOPEEK HA、AKSOPEEK CF、AKSOPEEK LCF